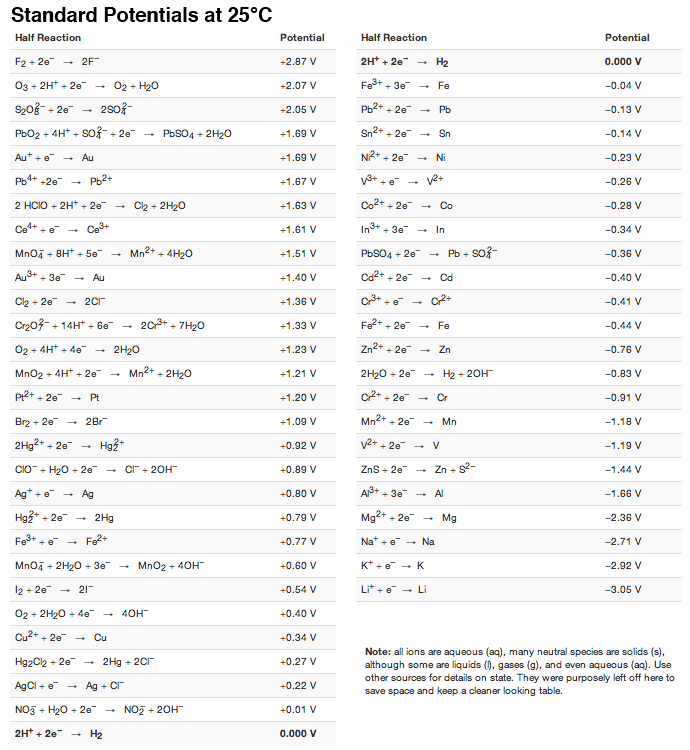
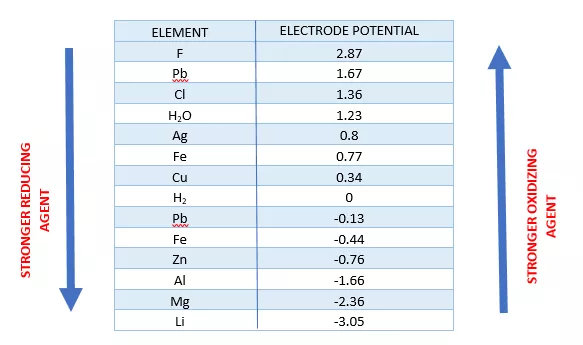
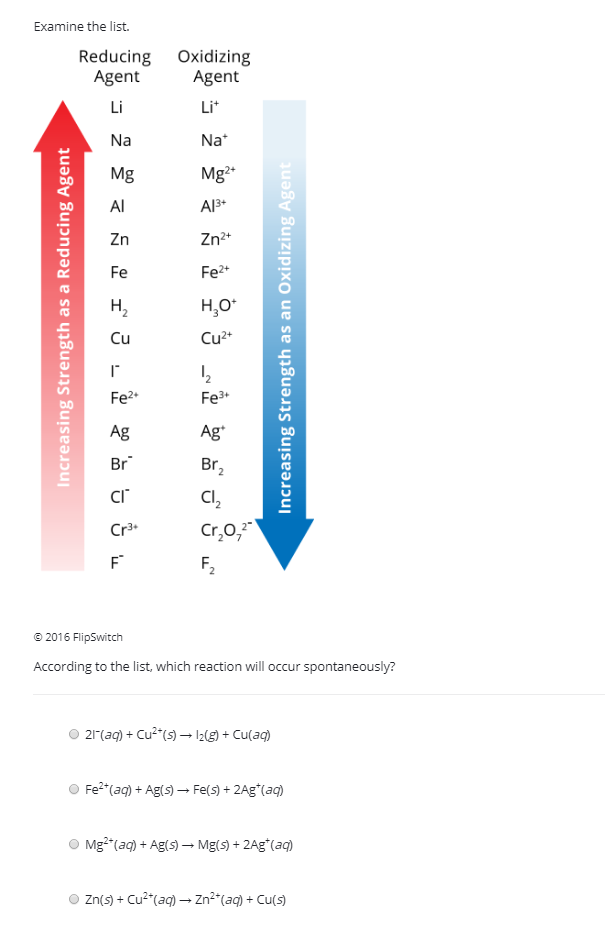
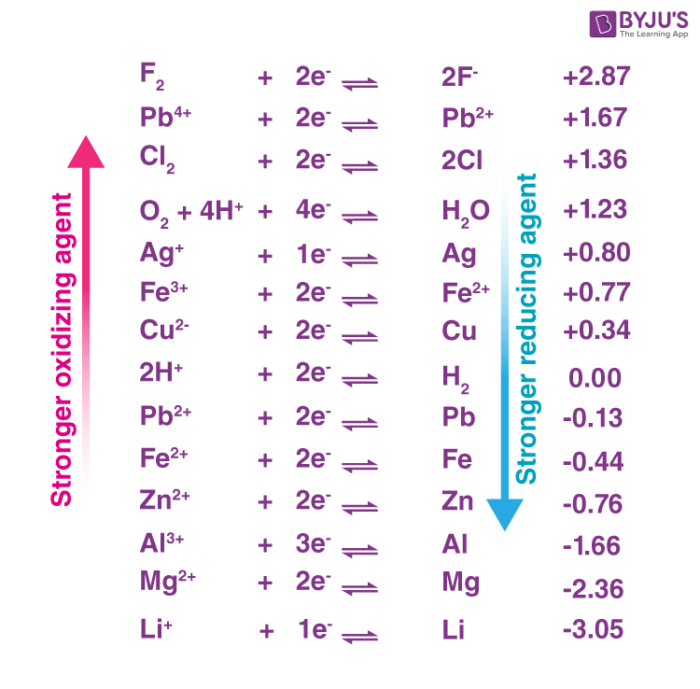
Reduction Reactions and Common Reducing Agents | Notas de química, Apuntes de clase, Fórmula química

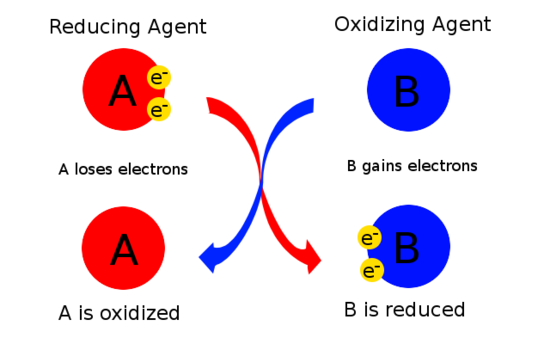
Difference Between Reducing Agent and Oxidizing Agent | Definition, Properties, Reaction Mechanisms, Examples

Oxidation of Food: What a Waste! Fruits and Vegetables oxidised when left in open air Solution: Seal in plastic wrap More radical: Add lemon juice to. - ppt download
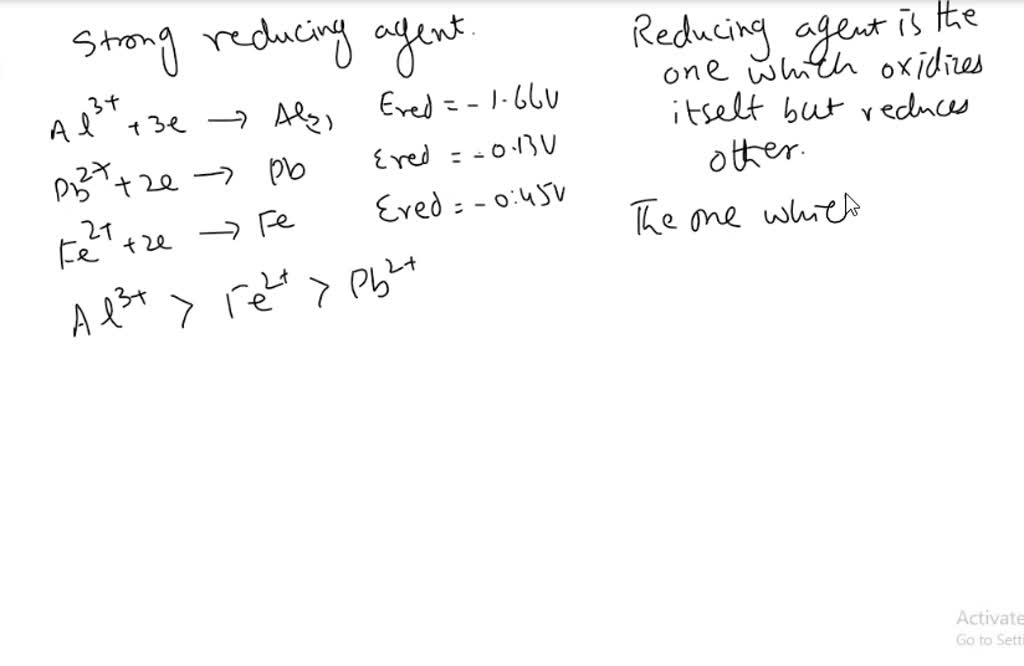
SOLVED: List the following reducing agents in order of increasing strength under standard-state conditions: Al(s), Pb(s), Fe(s)

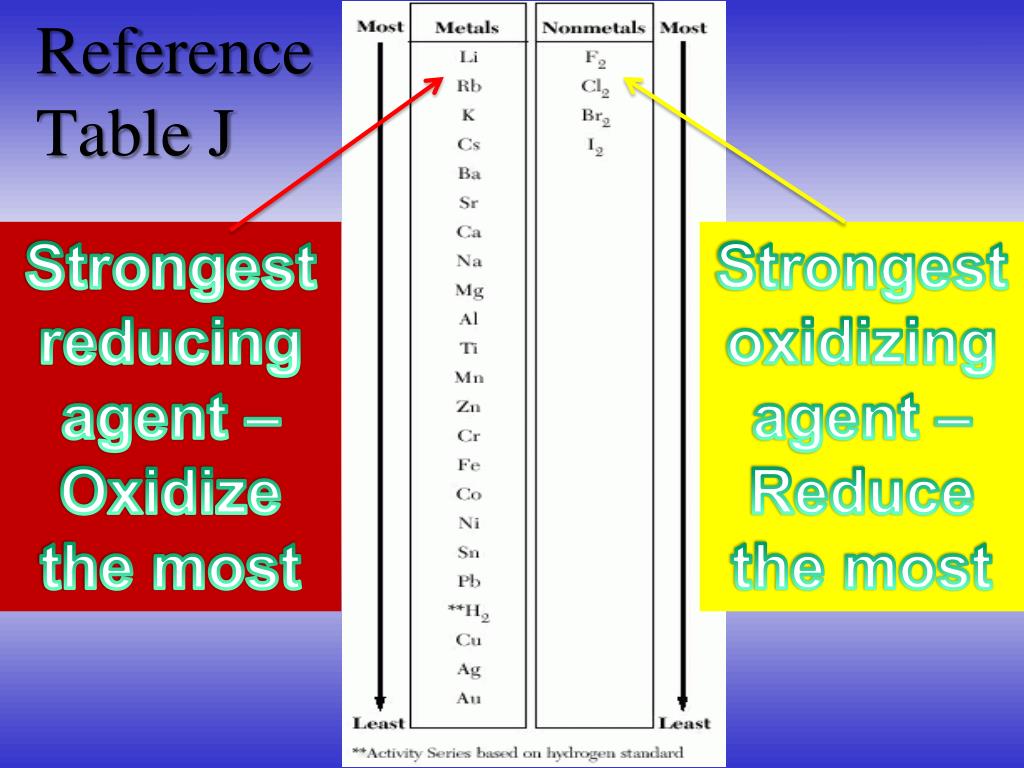
inorganic chemistry - Best oxidizing and reducing agents: Na, Zn^2+, Ba, Ba^2+, and Ag? - Chemistry Stack Exchange

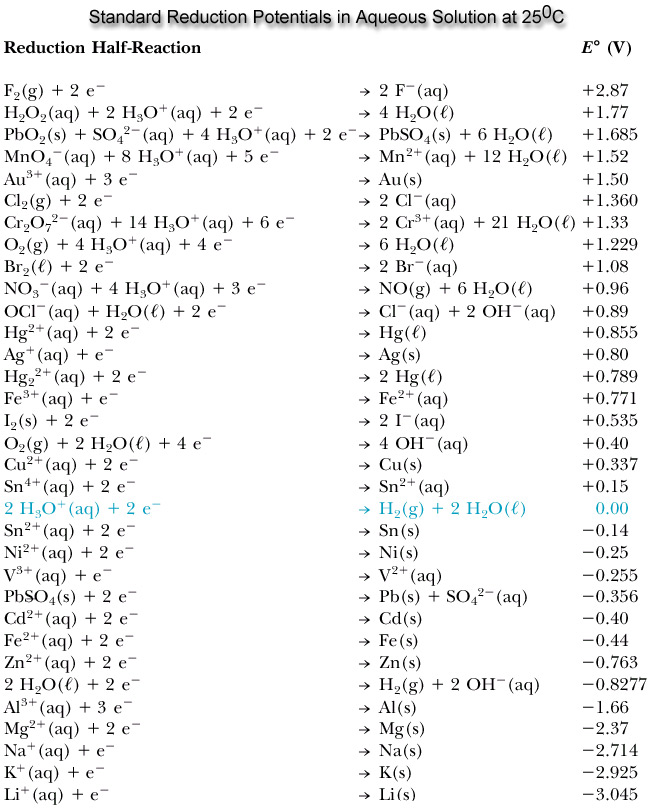


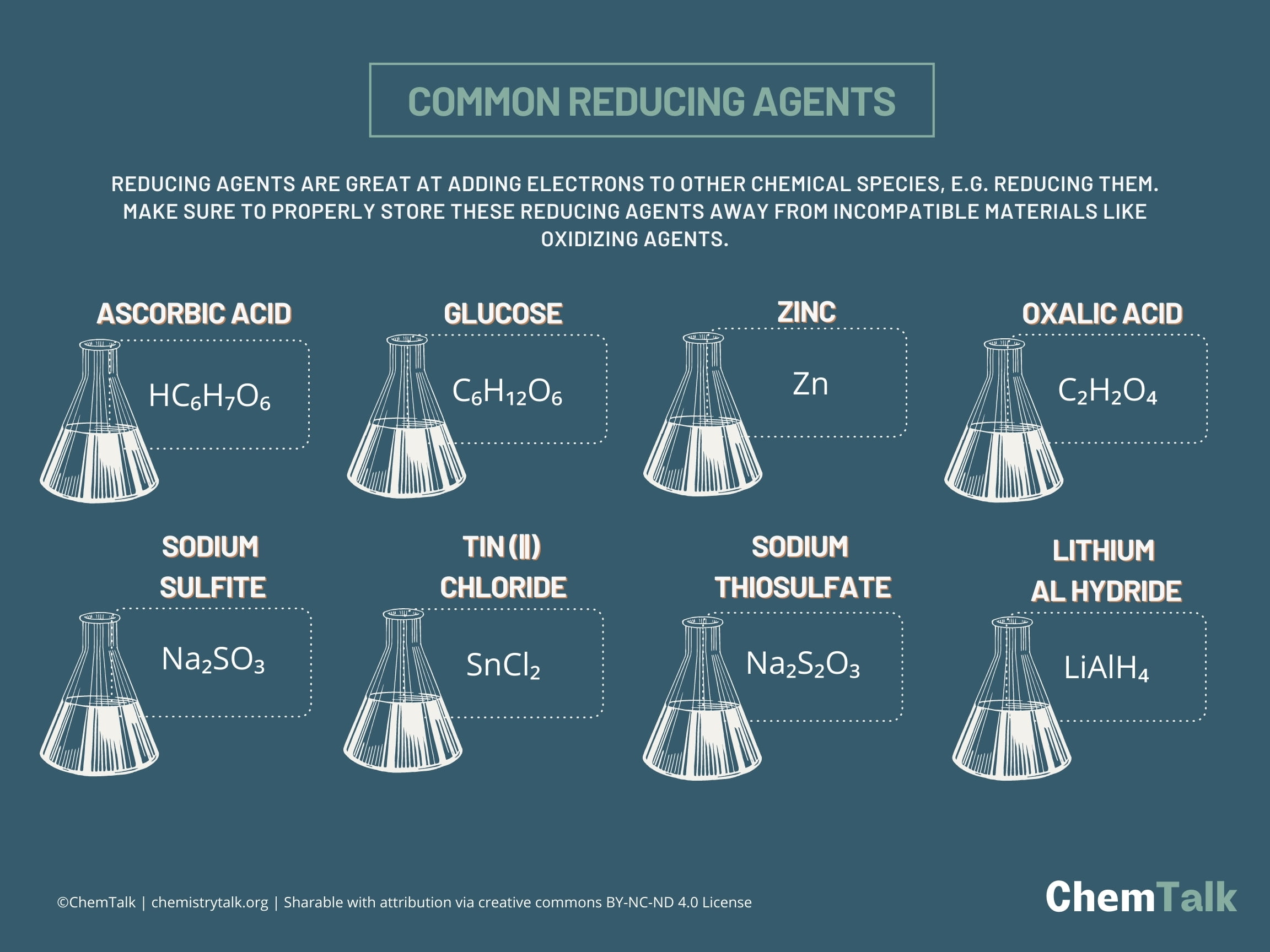
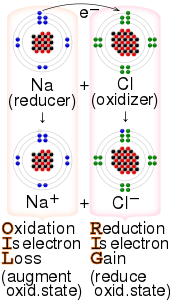

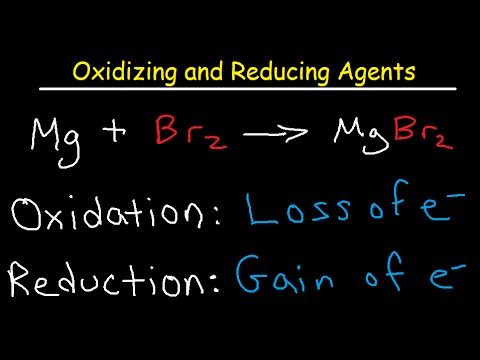
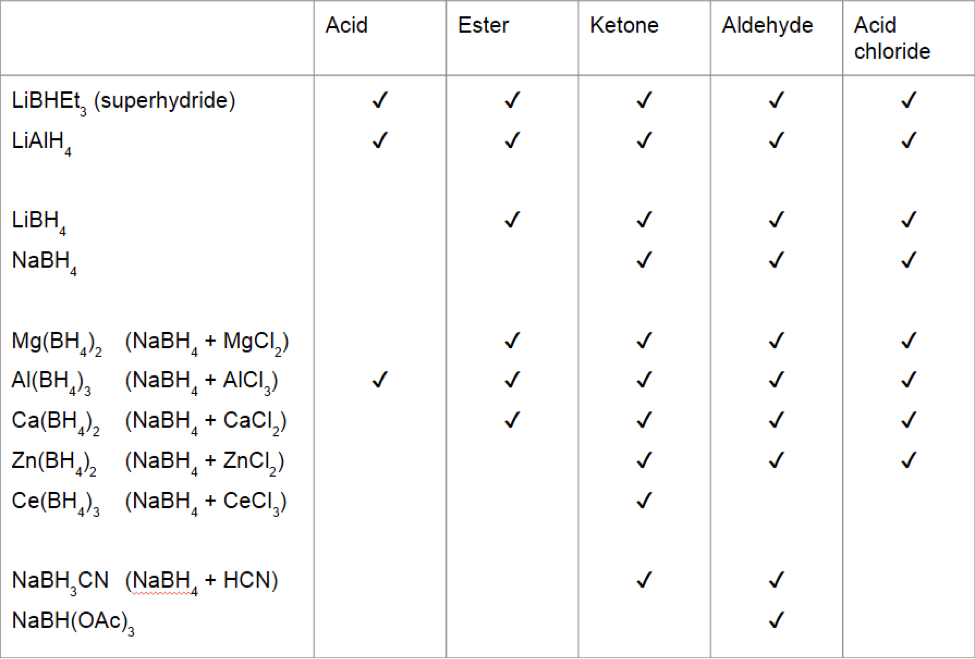
![AUFBAU1 [REFERENCE SECTION: REDOX POTENTIALS] AUFBAU1 [REFERENCE SECTION: REDOX POTENTIALS]](https://www.wissensdrang.com/media/tablerp.gif)